
Quality Commitment
We, BQ Plus, endeavour our full passion on the concerning of patient healthy. As an assure of patient safety, we establish our quality policy and devote ourself to make a safer world by providing better quality, innovative, and effective products, parts, and sterilization service. That’s a process never ends-we continually review our quality system and product against regulatory requirement to make sure we are on the right way.
We share our patients’ concerns and understand how deeply they need proper care, proper device when those are needed, which is why we always striving to do better and more when it comes to this important issue.
The quality policy bellow reflects our company’s focus on providing devices that patient can trust. We’ve worked hard to build and maintain this trust for many years.
- Delight our customers and deliver Better Quality promise.
- Design and deliver safe, excellent cost, and effective products.
- Comply with applicable regulatory & compliance requirements and enhance everyone’s duty for quality.
- Drive a culture of continuous improvement.

Mr. Jack Yang
BQ+ Vice GM & Management Representative
11-year Senior Auditor for TUV Sud
Certificates
BQ+ Medical has been Certified by TUV RL according to ENISO13485:2016 and MDD93/42/EEC. Also we are qualified EO sterilization service provider (holder of ISO11135:2014 certificate).
In China, we have aquired the CFDA certification on Anaesthetic masks.
Certificate ID: Shanghai Food and Drug Administration Production No. 20192519
- ISO13485: 2016


- MDD/93/42/EEC


- FDA

- KGMP

- Anvisa

We have a well qualified team….




The whole organization takes quality as our first priority.
- Total Quality Management
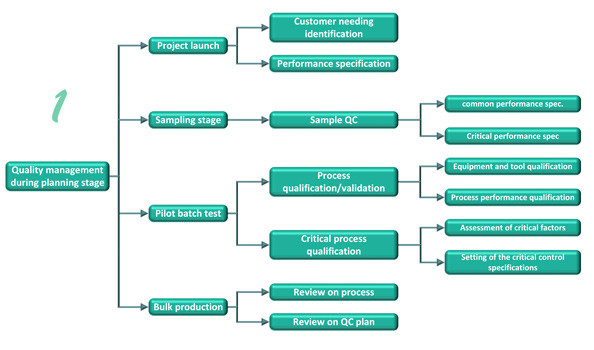
Planning

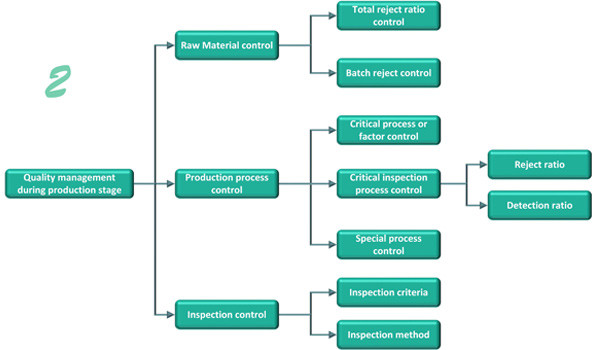
Production

Post-product


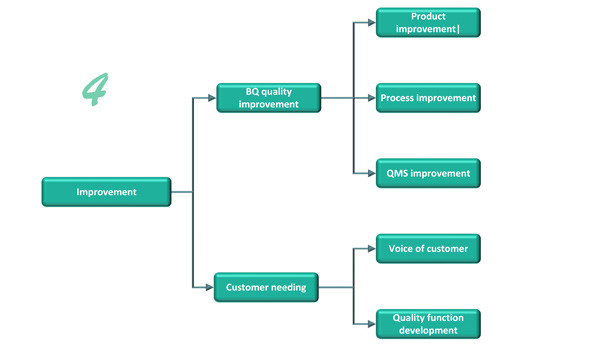
Improvement

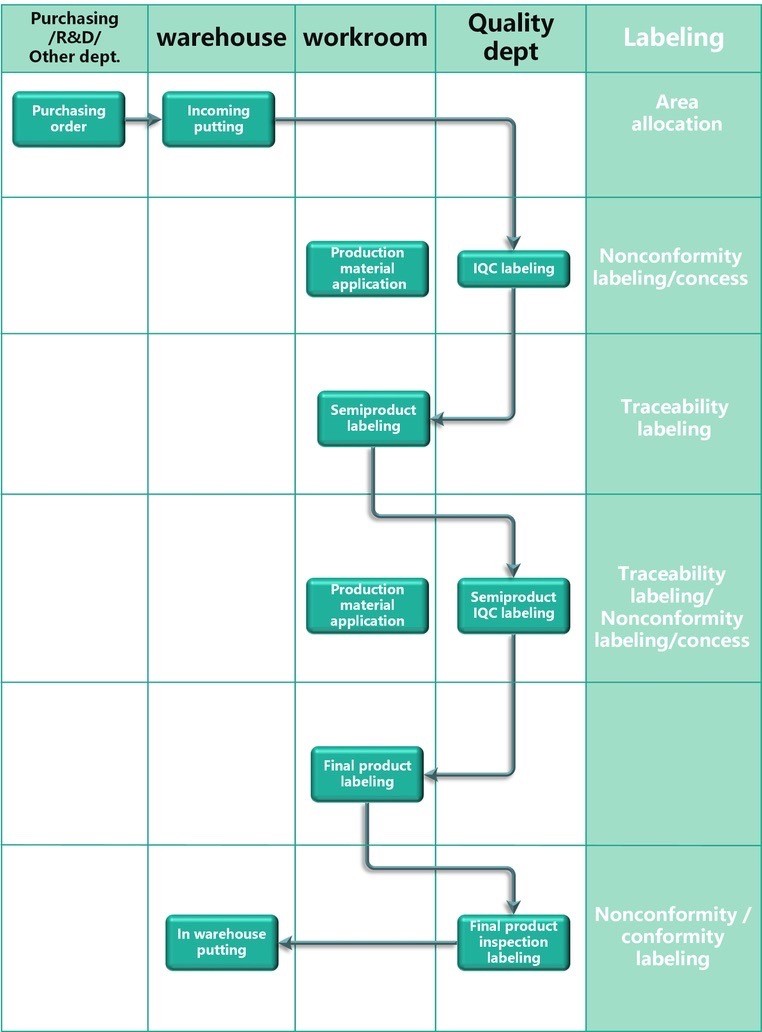
Traceability is one of the most important aspect for medical device and our QMS system, not only during the product realization stage, but during the whole device life cycle. For the purpose of protecting patient and avoid unlawfully misuse of the device, we care about and attach great importance to the traceability of our products. Handling of post market surveillance is one of important issue in our quality management system. Traceability ensures us to carry out our PMS system as we originally designed, through which risk can be minimized and patient benefit & customer’s interest are maximized.
DHR acts as central part in the whole traceability system during product realize stage. The DHR includes every process record, such as record for raw material inspection, manufacturing record according to planned schedule, process inspection and sterilization batch records etc., which is avaiable at request.
UDI system is under developing for the traceability of product during the whole lifetime of the product.
Traceability during product realization stage:
- Tel:
+86-021-57743953
- Address:
- No.18,Cheye Road,Songjiang District,Shanghai 201611 China.
- Email:
- info@bq-medical.com



